
Oncology Learning Collaborative Partnership
Some long-standing cancer diagnosis procedures are still used and often are required prior to approval of newer strategies. There are not a lot of barriers to getting these standard tests.

However, new and leading-edge diagnostics are rapidly entering the field. These include biomarker testing, artificial intelligence, and second opinion programs.
-
Biopsy
-
Bone scan
-
Complete blood count
-
CT scan
-
Circulating tumor markers
-
Cytogenic analysis
-
Immunophenotyping
-
MRI
-
Nuclear scan
-
PET scan
-
Ultrasound
-
Urine cytology
-
X-rays
Please see the glossary for more information on each test.
How to talk with cancer patients (Serious Illness Conversation Guide)
Stages of Cancer
Cancer staging is the process of determining the extent to which a cancer has grown and spread. A number from I to IV is assigned, with I being an isolated cancer and IV being a cancer that has spread from its origin.
For more information on staging, click HERE.
Biomarker Testing
Biomarker testing has taken hold in the last few years and is one of the best things to happen for someone who has developed cancer. Biomarkers, or substances such as proteins and genes found in blood, body fluids, or tissues, are useful in the diagnosis of:
- Type of cancer
- Staging level of growth (and therefore targeting the therapy that will have the best outcome for an individual patient)
- Measuring whether the treatment is working, or the cancer is returning
Testing is especially important for patients with rare cancers or those who have few treatment options, because mutations found in different cancers may overlap. If a person has a rare cancer with a mutation seen in other cancers, there may be a treatment that could potentially work in effectively treating the rare cancer as well.
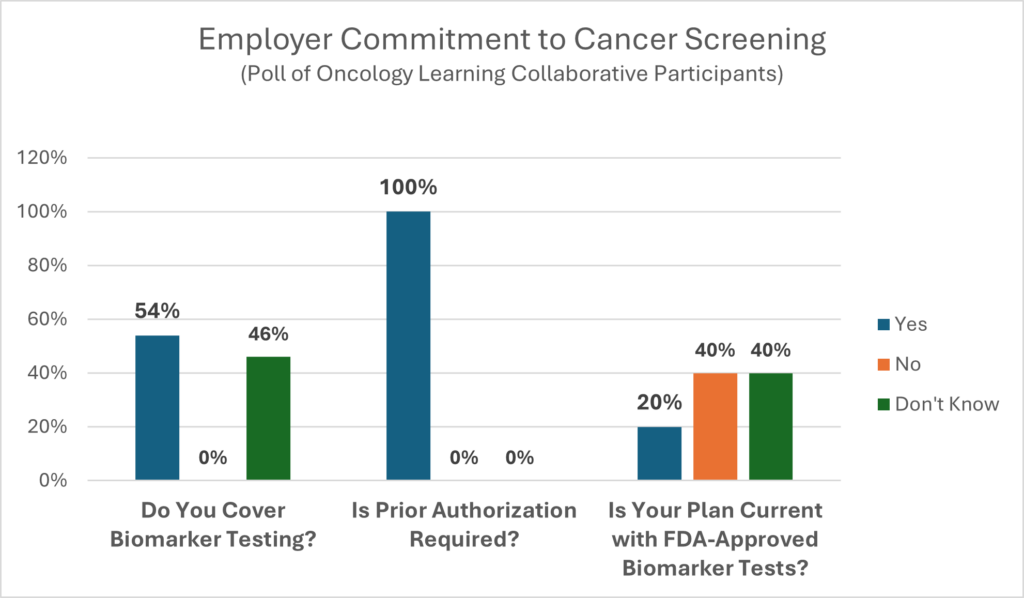
Biomarker testing allows oncologists to practice precision (personalized) medicine with the end results being saved lives, treatment, time, and money. As with most other rapidly emerging technologies, it is difficult for employers to keep pace with changes to assure their health benefits designs are promoting the best possible care for their members.
Biomarker testing is an area that is rapidly evolving. More than likely, carriers are covering biomarker testing, but with some barriers in the way. CancerCare’s comprehensive list of questions to ask your benefit consultants and carriers can be used to help close gaps in coverage and eliminate barriers to prompt and effective treatments. It includes questions on: coverage tied to guidelines, covered tests, test timing and utilization review, coverage limitations, interpreting results and coverage of ancillary services.
The toolkit also provides recommendations for biomarker coverage based on principles from the American Cancer Society Cancer Action Network (ACS CAN) model state legislation and may be useful in updating plan coverage.
Rosa Novo, Administrative Benefits Director at Miami-Dade County Public Schools
“We believe that biomarker testing is so important that we offer coverage without prior authorization to avoid delays in people getting appropriate care. The only time prior authorization comes into play is when the cancer is reoccurring, the person is on the organ transplant list and has been diagnosed with cancer, or when the patient has other chronic conditions. First, we immediately assign a care manager who coordinates all the care and services the person needs. This includes connecting members to community resources and automatically offering the behavioral health specialist, scheduling appointments, and providing information. Some of our PAs go through an appeals process. The Insurance carriers push back by saying there are so many of them with so many codes that it is hard to manage which can be a challenge. This has eliminated barriers to care, and we have increased the number of tests that don’t require the PA.”
Dan Dentzer, Manager of Health and Welfare Strategy, United Airlines
“The carriers are helping a lot. We look at our data to tell a story. The data tells us if the carrier is reaching out to members, helping members understand the benefits, directing them in the right way, and whether they are getting information over to our center of excellence (COE). We are also learning if they are working with our navigators to help the member throughout the overall process. The data also helps us do a better job of holding the carrier accountable as we use it to review the various cases to see what they did, what they could have done better, and what they did well. My recommendation is to stop using the standard or canned reports that are out there and design reports that tell you which biomarker tests you found to be helpful and where you need to go. For example, what were the number of tests approved, denied, approved if denied previously. It is important to review these reports and adjust as necessary. Also, rely on your consultants to help you ask the right questions. Hold your carriers accountable. Know how many tests they are doing, denying, or denying multiple times and then approving. Pay attention to the reporting.”
Lea Ann Biafora, CEO/Founder of Professional Cancer Care Experience Advisors, Beacon Advocates
“Providers are reluctant to order tests because of all the steps they must go through to get the approval. It is important that the carriers update in real-time for the tests and that the carriers approve the tests for maintenance. Cancer is smart and can mutate. The new technology may be able to identify mutations that were not identifiable before.”
The Midwest Business Group on Health’s employer action brief, “Biomarker Testing: What It Is and Why Employers Should Invest In It,” provides useful information about biomarker testing and the steps employers can take to ensure it is an available tool for precise and effective diagnosis and treatment of cancer.
EMPLOYER ACTION STEPS
Since use of biomarker testing to practice precision care for oncology is relatively new, it is important to make sure current medical and prescription drug benefits coverage is updated to include comprehensive coverage and eliminate unnecessary barriers to access. Assistance in understanding biomarker testing and assuring it is being adequately covered is provided in the “Employer Toolkit: Best Practices for Biomarker Testing Coverage (cancercare.org).”
- Confirm Coverage: Recognize that some insurance plans will not cover the costs of biomarker tests and will deem them “experimental and investigational.” This is more common in the fully insured plans than the self-insured plans. Check for outdated plan language to avoid overarching exclusions for genetic testing. Language should be modified to assure coverage for appropriate use is achieved and that members and call center representatives are alerted to check for prior authorization requirements for coverage of testing. Ensure that plan design language sections governing prior authorizations include specifics about any requirements around genetic and biomarker testing for diagnosis, treatment, and/or post-treatment monitoring. Confirm plan coverage of all tests that are FDA-approved as companion diagnostics to match patients to all FDA-approved targeted therapies and immunotherapies.
- Confirm Processes: Confirm with carriers that processes are in place to evaluate the use of biomarker testing efficiently and proactively for diagnosis, treatment, and ongoing monitoring.
- Keep Updated: Determine whether carriers are consistently evaluating the biomarker testing market and implementing opportunities for savings with specific lab networks and/or proactive use of panel tests over single biomarker testing.
- Monitor Clinical Communications: Ensure that carrier call center representatives are being given frequently updated lists or biomarker tests that do and do not require prior authorization reviews for coverage. To avoid delays in care, make sure they understand the plan language and benefits procedures.
- Verify Appeal Process: Confirm that your plan has a strategy in place to ensure easily followed directions for appeal filing, and that biomarker testing coverage appeals are processed and decisions are communicated without long delays. Check that appropriate clinical experts are involved in appeals reviews.
- Monitor the Testing Facilities: Determine whether there is a need for a narrow network of biomarker testing facilities that have the skills and resources to properly manage patient cases and control the scope and costs of these tests.
- Communicate Coverage: Consider implementing a member communication campaign to raise awareness of the value of biomarker testing and plan coverage of these tests.
Biomarker Testing: What it is and Why Employers Should Invest in It
Employers’ Prescription for Employee Protection Toolkit: Best Practices for Biomarker Testing Coverage
Employer Articles for Use of Biomarkers with Employees and Plan Members
National Cancer Treatment Alliance Biomarker Testing Toolkit – to view this resource contact lisa@flhealthvalue.org with a reference the material
Misdiagnosis
Many studies have been conducted to determine how often cancer is initially misdiagnosed and/or how often the patient is not put on the appropriate treatment plan. They range from 11% misdiagnosis to as high as 50% when the study combines misdiagnosis and inappropriate treatment. Second opinions have been one strategy to combat misdiagnosis, but there are many challenges for the patient, including cost, confusion about where to go for care, and concerns about offending a trusted physician. Some employers are looking at new programs that provide case reviews, saving the patient from chasing down a second opinion.
Jane Lutz, Senior Employer Account Executive at Genentech on behalf of Cody Adams, Benefits Manager
“Because we are an oncology company, we handle this differently than a typical program. We have all claims for newly diagnosed cancers go to AccessHope, because we want to make sure the person gets on the right drug at the right time. Typically, most employers take the approach where they send the claims for only the top five or so cancers to AccessHope.”
Second Opinion and Case Review
Many employers are opting to offer free second opinion programs for their members and encouraging their use as soon as possible after initial diagnosis. Given the rapidly advancing technology of cancer care, one of the greatest benefits of these programs is they provide members with peace of mind that their diagnosis is accurate, and their treatment plan is comprehensive.
Second opinion programs are especially helpful when a member is diagnosed with a rare or complex form of cancer as they provide opinions from COEs that specialize in treating it. These centers often have more advanced technology, more experienced oncologists, cutting-edge treatments that might not be offered elsewhere, or access to newer therapies through clinical trials. Programs may be provided by the medical plan carrier or through third-party vendors.
A significant percent of patients do not seek a second opinion because of cost, fear of delaying treatment (90% of breast cancer patients do not seek a second opinion), and concerns about damaging the relationship with the original provider.
Even so, given the rapidly advancing technology of cancer care, second opinions provide peace of mind that diagnoses are accurate and treatment plans are comprehensive. Second opinions are especially helpful with rare or complicated cases of cancer, as these second opinions typically come from centers of excellence with more advanced technology, more experienced oncologists, cutting-edge treatments, and access to clinical trials. Second opinion programs may be provided by the medical plan carrier or through third-party vendors. Often these programs are not used or are not as user-friendly as they should be.
REQUIREMENTS OF A SECOND OPINION PROGRAM
- Does the program provide actual engagement rates from current customers, the percentage of cases where the second opinion resulted in changes in diagnosis or treatment plan, and criteria used to determine it was a change?
- Is it easy for a member to use the program – one call does it all?
- Does the program provide one point of contact for the member who helps them navigate through the process and answers their questions?
- Will the program provide post-opinion discussions of results for members and treating physicians?
- Is the program designed with flexible pricing strategies, PMPM, or per-case administrative costs?
Rosa Novo, Administrative Benefits Director at Miami-Dade County Public Schools
“We cover second opinions with prior authorizations. We have two large health systems with cancer centers that rely on physicians having team discussions and multiple protocols in addition to AI. The Miami Cancer Institute and the Sylvester Comprehensive Cancer Center call these team discussions physician huddles, and they meet first thing in the morning. A report on individual cases is delivered to the case manager at Cigna and Cigna reports each case, deidentified, to us. They will also bring in other specialists, if necessary.”
Sherri Samuels-Fuerst, VP of Total Rewards, Sargento Foods
“You need to remind everyone that these second opinion programs are available. It is critical and helpful to make sure you have all the information embedded in as many other resources as possible. Then, regardless of where they bump into a resource, they will be reminded and encouraged to use the service. We have had two people who were diagnosed with cancer, end up not having it, so having the right diagnosis is imperative.”
Dan Dentzer, Manager of Health and Welfare Strategy, United Airlines
“We have a second opinion center-of-excellence program and we have found it helps give an employee reassurance that what they are having done is correct. Secondly, it saves the plan money. This is a win-win. Physicians are happy to get a consultation from City of Hope, Northwestern, or Mayo Clinic.”
Artificial Intelligence to Diagnose and Treat Cancer
Artificial Intelligence (AI) for diagnosing and treating cancer depends on computer programs and algorithms. AI has been used for over 20 years to analyze mammograms and is now being used to diagnose and treat cancers often missed in early stages and hard to recognize with the naked eye like pancreatic, prostate, lung, and skin cancers.
Public attitudes about the use of AI in healthcare significantly lean toward the side of caution. For example, a Pew Research survey revealed Americans feel uneasy about AI’s role in their health care with 60% of 11,004 adults saying they would be uncomfortable if their healthcare provider used AI for tasks like diagnosing diseases and recommending treatments, while 39% indicated they would feel at ease. When it comes to improving treatment outcomes, 38% think that AI in health and medicine would lead to better overall outcomes for patients, 33% think it would lead to worse outcomes, and 27% think it would not have much effect.
Employers should inquire about what is and is not being covered when it comes to AI. Select AI systems have been covered on a per-use basis by some payers since 2020, but there is concern that per-use AI reimbursement may result in overuse. Alternative reimbursement approaches are being developed such as outcome-based payments.
Two examples of AI programs are:
- SmartLinQ: When an oncology practice participates in the program, patient information patients is transferred to a platform where the data is used to help inform diagnoses and care decisions.
- Sibyl: MIT and Mass General Cancer Center developed the AI program “Sybil.” Sybil was trained on low-dose chest computed tomography scans, for those between ages 50 and 80 who either have a significant history of smoking or currently smoke. For patients undergoing screening for lung cancer, Sybil is able to look at an image and accurately predict the risk of a patient developing lung cancer within six years.
HHS EXECUTIVE ORDER 10-30-23
An HHS AI Task Force is developing a strategic plan with appropriate guidance to include policies and frameworks with integrated regulations as needed. Focus will be on responsibly deploying and using AI and AI-enabled technologies in the health and human services sector, spanning research and discovery, drug and device safety, healthcare delivery and financing, and public health. Three areas being addressed include:
AI Equity. Requires active monitoring of the performance of algorithms to check for discrimination and bias in existing models to identify and mitigate any discrimination and bias in current systems.
AI Security. Mandates integration of safety, privacy, and security standards throughout the software development lifecycle, with a specific aim to protect personally identifiable information.
AI Oversight – Ensures appropriate human oversight over the application of AI-generated output from the development, maintenance, and utilization of predictive and generative AI-enabled technologies in healthcare delivery and financing.
Two questions to ask medical carriers:
- Does your plan cover the use of artificial intelligence programs?
- If yes, is prior authorization required for coverage?
Kenneth Aldridge, Director of Health Services, Rosen Hotels and Resorts
“We are moving into a new population health, practice analytics, predictive modeling and AI system to gather actionable data, particularly on lung cancer.”
© Copyright 2024 Central Florida Health Care Coalition, Incorporated d/b/a Florida Alliance for Healthcare Value
The Central Florida Health Care Coalition, Incorporated d/b/a Florida Alliance for Healthcare Value is providing this information to our employer members solely in our capacity as a 501c3 nonprofit education organization and not as advice in any capacity. The information that is not in the public domain is private and confidential.
